Running Update:
|
1/4/2017
|
Run
|
|
5.0 mi
|
48:29
|
|
1/6/2017
|
Run
|
|
5.0 mi
|
47:50
|
|
1/8/2017
|
Run
|
|
5.0 mi
|
48:44
|
|
1/9/2017
|
Swim
|
|
1450.0 m
|
|
|
1/11/2017
|
Run
|
|
5.0 mi
|
47:10
|
|
1/13/2017
|
Run
|
|
5.0 mi
|
47:46
|
|
1/14/2017
|
Swim
|
|
1200.0 m
|
|
|
1/15/2017
|
Run
|
|
7.5 mi
|
1:16:04
|
|
1/16/2017
|
Swim
|
|
1700.0 m
|
|
|
1/18/2017
|
Swim
|
|
1750.0 m
|
|
|
1/18/2017
|
Run
|
|
5.0 mi
|
49:23
|
|
1/20/2017
|
Run
|
|
5.0 mi
|
47:59
|
|
1/22/2017
|
Run
|
|
7.5 mi
|
1:14:23
|
|
1/23/2017
|
Swim
|
|
1600.0 m
|
|
|
1/25/2017
|
Run
|
|
7.6 mi
|
1:14:23
|
Current
events have taken precedence (!) to my blogging lately, but I’m back with an
update today, since NF isn’t going away anytime soon. I’ve slowly started preparing for my spring
races. You’ll notice I’ve added swimming
to my training! I joined a Master’s Swim
group this fall in the hope of improving my running performance. We’ll see.
Here
is my official 2017 race schedule to support the Children’s Tumor Foundation:
NYC Half Marathon—Sunday, March 19th
Vermont City Marathon—Sunday, May 28th
New Haven Road Race Half Marathon—Monday,
September 4th
Marine Corps Marathon—Sunday, October 29th
As always, our official fund raising site is www.KRath4Jane.com and it has been updated to reflect the 2017 events.
NF Update:
The
New England Journal of Medicine (NEJM) is one of the oldest and most
prestigious peer-reviewed medical journals.
Last month this journal
published the results of phase I of the clinical trial in which Jane is
participating. Jane was “Patient 24”, and you can actually
see the plot of her tumor volume in the article. How amazing is that??
The
day the article came out I showed Jane a copy of the journal. I told her the NEJM is a famous medical
journal that I read (I have a subscription, so she has seen the journal before
around our house). I showed her the
cover, which lists the articles, and I pointed out the article with
“neurofibromatosis” in the title. I told
her it was an article about her medicine,
written by her doctors, and that it
included information about her! She
plunked right down at the kitchen table and began to read through it.
The
full article is available only to subscribers, but you can see the abstract
here.
Or look for it in the library: N Engl J Med 2016; 375:2550-256.
I
pointed out that the article summarized the results of the first 24 children to
take her medicine. She asked, “Where
does it say about the 24 children?” so I showed her the results section of the
abstract. She read “A total of 24
children (median age, 10.9 years; range, 3.0 to 18.5)…” I told her that meant the average age to the
kids was about 11 years, that the youngest child was 3 and the oldest was
18-1/2. She flipped to Table 1 and
started to read it line by line, following with her finger.
 |
| The New England Journal of Medicine ©2017 |
The quotes are Jane’s as she read it through:
“Number
of patients…24”
“Median
age…10.9”
“13
boys and 11 girls. That’s
interesting—there were more boys than girls.”
“Number
of patients who had previous medical interventions…19” I said that means the
number of kids who had taken other medicines for their tumors before this
one. “I’m one of them.”
“Number
of previous medical interventions for treatment of plexiform
neurofibroma…41. That means 19 kids had
41 different medicines. How many
medicines did I have?” I told her first
she had shots (Pegintron), then she had the medicine from Indiana
(Gleevec). “So, I had two.” She noted that that was the same as the
median number of previous medical interventions per patient (2).
“Number
of previous debulking surgeries…” I told
her that meant how many children had had surgery for their tumor. “Not me.”
“Predominant
target location of plexiform neurofibroma.
Face…4. That’s me!” She went on to read that one patient had a
head and neck tumor, 6 had a neck and chest tumor, 4 had a truncal tumor, 8 had
a truncal and extremity tumor, and 1 had a whole-body tumor. I commented that it didn’t seem to be enough
patients, but she quickly added them up and said, “Yup. It adds up to 24.”
“Progression
status. What’s that?” I said that “progressive” was another name
for “growing”, and that her tumor was growing when we started this trial. “Mine was ‘nonprogressive’ before, when I
took the shots. It was not growing and
not shrinking.” I said she was
correct—the interferon had kept her tumor stable for about a year before it
became “progressive” again.
“Documented
plexiform neurofibroma-related complication at baseline.” I told her it was complications, or problems,
caused by the tumor before starting the medicine. “Oh!” she lamented, “One child had vision
loss!” Then, “What’s ‘motor
dysfunction’?” I told her it meant
difficulty moving, for example, moving arms or legs. We noted that her friend, Travis, who has a
PN in his leg, has motor dysfunction from his tumor. I pointed out, however, that Travis was not
in this particular paper, because he was not in the original group of 24
children. “Will there be another article
in the magazine after the next 24 children?”
I told her probably. She then
started counting by 24: an article after 48 children, then after 72 children,
then after 96 children… :)
“Pain…13. I don’t have any pain.”
“Disfigurement. Well, I have some, I think. What does ‘disfigurement’ mean?” I said it’s when something changes shape,
like her cheek changed shape a bit because of her tumor. She agreed.
With
Table 1 done, she turned right to Table 2.
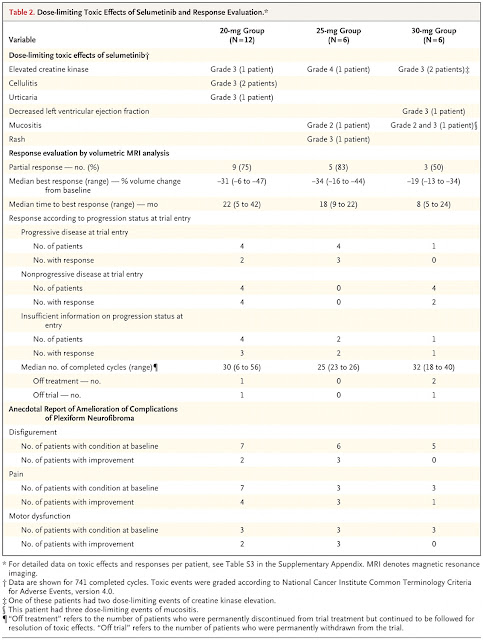 |
| The New England Journal of Medicine ©2017 |
“Dose-limiting toxic effects of selumetinib.” I told her that meant side effects that were so bad that the child had to stop the medicine. “I had to stop it once.”
I
explained what elevated creatine kinase, cellulitis, urticaria, decreased left
ventricular ejection fraction, and mucositis were. Then she read, “Rash…1 patient. That’s me!
Maybe people will read this and wonder who was the child who got the
rash!” Then she puzzled, “It says ‘Grade
3’, but I was in grade 1 when it happened.”
I explained that “Grade 3” was referring to the severity of the rash,
not her school grade :)
 |
| Figure 2. The New England Journal of Medicine ©2017 |
Figure 2A: “Which
one am I?” Number 24. She got a ruler
and drew a line from her bar to the y-axis labeled “Percent Change in Tumor
Volume” and proclaimed, “It’s halfway between -30 and -40, so it’s about
-35.” I told her she was correct: the
maximum decrease in her tumor volume was measured at 33%.
Figure
2C: “Which
one am I?” I pointed it out—hers is the black plot in the middle graph of
Figure 2C (25mg/m2)—and described the various sections: pre-medicine,
Pegintron, Gleevec, selumetinib.
“I
feel bad for patient 3 and patient 8.
Their tumors kept growing.” She
said, “Mine grew a tiny bit,” pointing to a brief plateau in her tumor volume
after 18 months on the trial. At first
Jane thought that must correspond to the month she was off the medication. “How long does it take for the medicine to
kick in again?” I told her I didn’t
know. She answered herself confidently:
“Probably a week. So, that’s one month
plus one week… Would that cause it to
grow?” I told her the more likely reason
for the plateau was that it was around this time that she went from sedated to
non-sedated scans, which would affect the way the tumor was measured.
 |
| Figure 3. The New England Journal of Medicine ©2017 |
Figure
3: “Does
it say my name? Does it have my
picture?” I explained that medical
journals don’t use patients’ names or pictures of their faces in order to
maintain the patients’ privacy. “What if
the tumor is on your face?” She answered
herself, “They could just cover part of it, like this.” She covered her eyes and forehead with her
hands. I agreed, and said the journals
often use a black bar to cover a patient’s eyes.
I confess seeing the study in print in
a prominent medical publication has caused me mixed emotions: on the one hand,
I am thrilled that we got a chance to participate in the study and that it’s
had such good results. I’m also tickled that Jane is “published” in the
NEJM! On the other hand, it reminds me that Jane was one of only 24
“guinea pigs” for the study, and that she has a unique enough medical condition
to warrant a report in the NEJM.
I have allowed myself to envision,
maybe 10-15 years from now, Jane and the other 23 kids reuniting at NIH to
celebrate when selumetinib finally gets FDA approval and becomes the
standard-of-care for plexiform neurofibromas. Maybe she’ll be starting
medical school at that point :)

No comments:
Post a Comment